FDA approval of Tarpeyo for primary immunoglobulin A nephropathy drives stock gains
- Calliditas Therapeutics shares rise 33% after FDA approves Tarpeyo
- Tarpeyo approved to treat certain patients with primary immunoglobulin A nephropathy
- Stock hits 52-week high of $25.64 on May 8
- Tarpeyo is the first fully FDA-approved treatment for IgAN based on a measure of kidney function
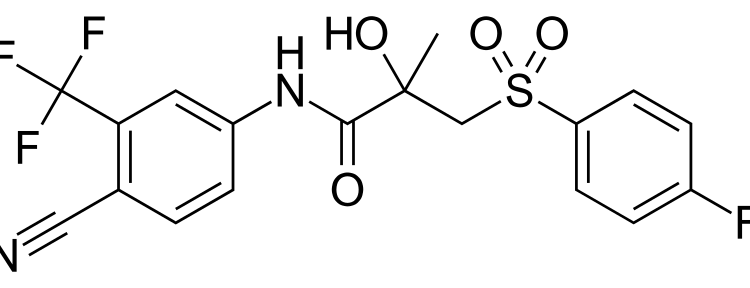
Shares of Calliditas Therapeutics soared 33% in late-trading after the U.S. Food and Drug Administration (FDA) approved Tarpeyo for the treatment of certain patients with primary immunoglobulin A nephropathy (IgAN). Tarpeyo, also known as budesonide, delayed release capsules, is the first fully FDA-approved treatment for IgAN based on a measure of kidney function. The stock reached a 52-week high of $25.64 on May 8. This FDA approval marks a significant milestone for Calliditas Therapeutics and offers hope for patients at risk for disease progression in IgAN.
Public Companies: Calliditas Therapeutics (N/A)
Private Companies:
Key People:
Factuality Level: 8
Justification: The article provides factual information about Calliditas Therapeutics and the FDA approval of Tarpeyo to treat primary immunoglobulin A nephropathy. The information is specific and does not contain any obvious bias or opinion. However, the article is short and lacks in-depth analysis or additional context.
Noise Level: 7
Justification: The article provides some relevant information about Calliditas Therapeutics and the FDA approval of Tarpeyo for the treatment of primary immunoglobulin A nephropathy. However, it lacks in-depth analysis, scientific rigor, and evidence to support its claims. The article also does not provide any actionable insights or explore the consequences of the FDA approval on the company or patients. Overall, it contains some noise and lacks depth.
Financial Relevance: Yes
Financial Markets Impacted: Shares of Calliditas Therapeutics
Presence of Extreme Event: No
Nature of Extreme Event: No
Impact Rating of the Extreme Event: No
Justification: The news article pertains to the approval of a drug by the FDA, which can have financial implications for Calliditas Therapeutics and its stock.
 www.marketwatch.com
www.marketwatch.com