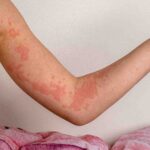
Clinical-stage biotech company achieves key goal in severe chronic spontaneous urticaria
- Celldex Therapeutics shares soar over 30% in premarket trading
- Phase 2 study of barzolvolimab shows success in severe chronic spontaneous urticaria
- Significant mean change from baseline to week 12 compared to placebo
- Limited treatment options for chronic spontaneous urticaria patients
- Barzolvolimab shows favorable safety profile
Celldex Therapeutics shares experienced a significant surge of over 30% in premarket trading following the announcement of positive results from a Phase 2 study of their lead product candidate, barzolvolimab. The study focused on patients with moderate to severe chronic spontaneous urticaria refractory to antihistamines, including those who had previously received biologics. The primary efficacy endpoint was met, with a statistically significant mean change from baseline to week 12 compared to a placebo. This is particularly significant as treatment options for chronic spontaneous urticaria patients are limited, especially for those who do not respond to omalizumab, the only approved therapy currently available. Furthermore, barzolvolimab demonstrated a favorable safety profile, paving the way for further development in Phase 3 studies.
Factuality Level: 8
Factuality Justification: The article provides specific information about Celldex Therapeutics’ Phase 2 study of barzolvolimab and its positive results in treating severe chronic spontaneous urticaria. The information is presented in a straightforward manner without any obvious bias or opinion. However, the article lacks additional context or expert opinions to fully evaluate the study’s significance and potential limitations.
Noise Level: 7
Noise Justification: The article provides information about Celldex Therapeutics’ Phase 2 study of its lead product candidate barzolvolimab in severe chronic spontaneous urticaria. It mentions the increase in Celldex shares and provides details about the study and its results. However, the article lacks scientific rigor and intellectual honesty as it does not provide any specific data or evidence to support the claims made. It also does not explore the consequences of the study results on patients or the potential risks involved. Overall, the article contains some relevant information but lacks depth and critical analysis.
Financial Relevance: Yes
Financial Markets Impacted: Celldex Therapeutics
Presence Of Extreme Event: No
Nature Of Extreme Event: No
Impact Rating Of The Extreme Event: No
Rating Justification: The article pertains to a clinical-stage biotechnology company, Celldex Therapeutics, and its positive Phase 2 study results for its lead product candidate. There is no mention of any extreme event or its impact.
Public Companies: Celldex Therapeutics (N/A), Novartis (N/A)
Key People:
 www.marketwatch.com
www.marketwatch.com