FDA Boosts Development of Innovative Cancer Therapy
- FDA grants orphan drug and rare pediatric disease designations for Cellectis’ UCART22 treatment
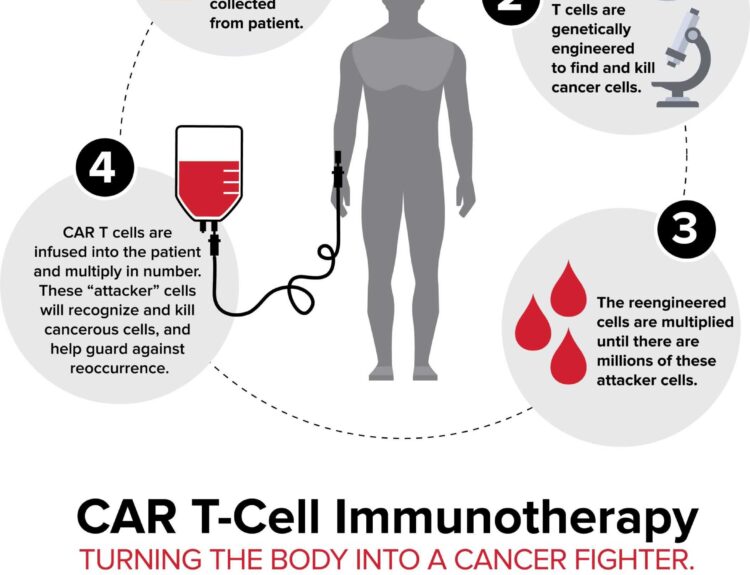
- UCART22 is a CAR T-cell therapy targeting acute lymphoblastic leukemia (ALL)
- Orphan drug status can speed up development and reduce costs
- Rare pediatric disease designation may lead to priority review voucher
- 1,560 U.S. deaths related to ALL in 2022
- Clinical data presented in December showed promising results
The U.S. Food and Drug Administration (FDA) has granted orphan drug and rare pediatric disease designations to Cellectis’ candidate, UCART22, for treating acute lymphoblastic leukemia (ALL). This treatment affects fewer than 200,000 people. The orphan drug status can expedite development and reduce costs. Rare pediatric disease designation may lead to a priority review voucher. Cellectis highlighted the urgent need for treatments for patients not eligible for other therapies. In the U.S., 10% of leukemia cases are ALL, with 1,560 deaths in 2022. Encouraging clinical data was presented in December, and an update on UCART22’s progress is expected by year-end.
Factuality Level: 10
Factuality Justification: The article provides accurate information about the FDA’s designations for Cellectis’ drug candidate, explains the benefits of these designations, gives relevant background information on ALL and UCART22, and presents a positive outlook on the clinical data. It is objective and free from sensationalism or personal opinions.
Noise Level: 1
Noise Justification: The article provides relevant information about the FDA’s designations for Cellectis’ UCART22 drug candidate for treating acute lymphoblastic leukemia, including its potential benefits and the urgent need for treatments in this area. It also mentions the encouraging clinical data presented in December and expected updates by year-end. However, it lacks analysis or exploration of broader implications and does not offer actionable insights.
Public Companies: Cellectis (CLLS)
Key People: Mark R. Long (Author)
Financial Relevance: Yes
Financial Markets Impacted: Biopharmaceutical industry
Financial Rating Justification: The article discusses the FDA granting orphan drug and rare pediatric disease designations to Cellectis’ candidate for treating acute lymphoblastic leukemia, which could impact the development and approval process of their UCART22 drug. This is relevant to financial markets as it affects the biopharmaceutical industry and potentially impacts the company’s stock value and future revenue.
Presence Of Extreme Event: No
Nature Of Extreme Event: Other
Impact Rating Of The Extreme Event: No
Extreme Rating Justification: There is no mention of an extreme event in the article.
 www.marketwatch.com
www.marketwatch.com