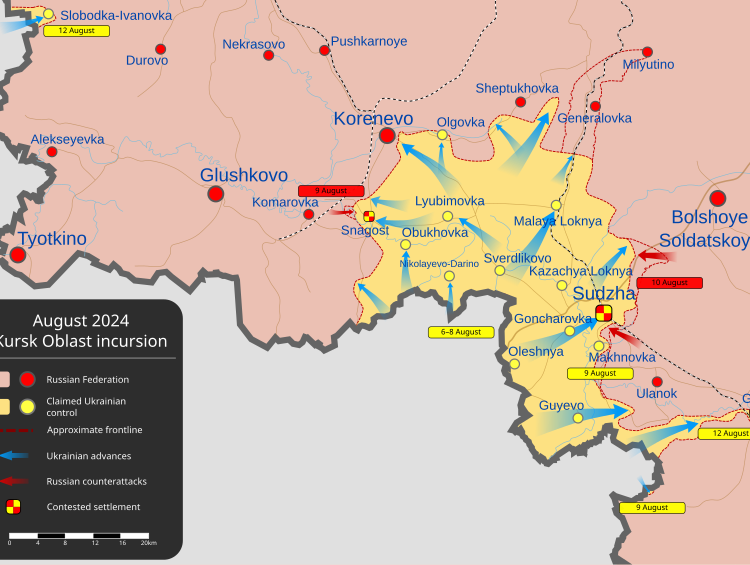
A setback in the quest to legalize psychedelics
- FDA rejects ecstasy-based drug for PTSD treatment
- Decision a setback for decades of efforts to legalize psychedelics
- Veterans and advocates disappointed
- Concerns raised about safety and efficacy studies
The FDA has rejected an ecstasy-based drug, MDMA, as a treatment for post-traumatic stress disorder (PTSD), dealing a blow to decades of efforts to legalize psychedelics and disappointing veterans’ advocates who were seeking better medicine for the 13 million Americans suffering from PTSD. The decision comes after FDA staff and expert advisers raised concerns about the safety and efficacy studies conducted by Lykos Therapeutics.
Factuality Level: 6
Factuality Justification: The article provides accurate information about the FDA’s decision on MDMA-based drug and its implications for PTSD treatment, but it lacks some details and could be more objective in presenting the situation.
Noise Level: 6
Noise Justification: The article provides some relevant information on the FDA’s decision regarding MDMA-based drugs and their potential use in treating PTSD, but it lacks a comprehensive analysis of the long-term implications or possible solutions. It also does not offer much insight into the reasons behind the FDA’s decision or explore the consequences for those affected by this choice.
Private Companies: Lykos Therapeutics
Key People: Liz Essley Whyte (Writer)
Financial Relevance: No
Financial Markets Impacted: This article does not impact financial markets or companies.
Financial Rating Justification: The article discusses the FDA’s decision regarding an ecstasy-based drug for PTSD treatment, which is a medical and social issue rather than a financial topic. It does not mention any direct impact on financial markets or specific companies.
Presence Of Extreme Event: No
Nature Of Extreme Event: Other
Impact Rating Of The Extreme Event: Minor
Extreme Rating Justification: There is no extreme event mentioned in the article, but the rejection of the MDMA-based drug by the FDA can be considered a minor setback for the efforts to legalize psychedelics.
Move Size: No market move size mentioned.
Sector: Healthcare
Direction: Down
Magnitude: Medium
Affected Instruments: Stocks
 www.wsj.com
www.wsj.com